Case Study 1: Selection for the Fe2O3 - H2O chemical system
The selection for Fe,cr (entropy), Fe2+ and Fe3+ (entropy and formation enthalpy) is taken from Parker and Kodakhovsky (1995), consistently with the CODATA89 selection. The selection is verified against magnetite and hematite solubility. The speciation model is refined after the available complexation data and it is finally assessed against ferrihydrite solubility. Full details in Blanc et al. (2012)
Step 1. Fe,cr, Fe2+ and Fe3+ properties assessment, the hematite solubility.
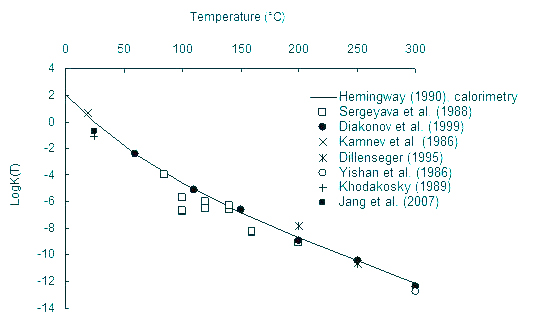
Hematite (Fe2O3) equilibrium constant as a function of temperature.
Step 2. Refinement of the speciation model for the Fe2O3-H2O system.
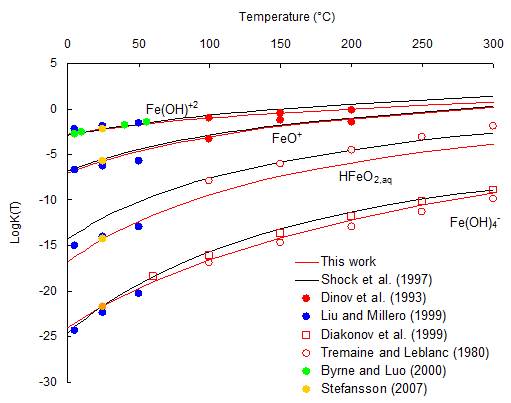
FeIII hydrolysis constants as a function of temperature (in red, refined values)
Step 3. Verification against ferrihydrite solubility.
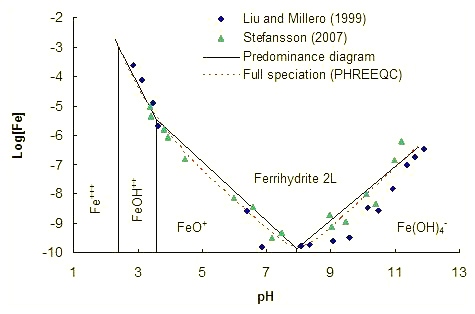
Ferrihydrite solubility at 25°C.
The refinement process is then extended to the other systems of interest.
Dernière mise à jour le 10.07.2020