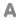Formula : Al
Family:
Space group:
Isometric - Hexoctahedral H-M Symbol (4/m 3 2/m) Space Group: F m3m,P m3m
Dana class:
1.1.1.5 (1)Native Elements(1.1) with metallic elements other than the platinum group (1.1.1) Gold group
High temperature:
Minor phase in low oxygen fugacity environments. In volcanic environment. Aluminum is present in some solid waste from incineration. Aluminun is a strong oxydizer.
Geological context:
No data are avalaible for the natural environment. Produced from chemical extraction of bauxite (Al2O3).
Dernière mise à jour le 25.01.2021