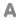Formula : (Na0.044K0.762)(Si3.387Al0.613)(Al1.427Fe0.376Mg0.241)O10(OH)2
Family:
Space group:
Monoclinic - Prismatic HM symbol (2/m) Space Group: C 2/m
Dana class:
71.2.2d.2VIII- 71 Phyllosilicate Minerals ; 71.2 Phyllosilicates Phyllosilicate Sheets of Six-Membered Rings with 2:1 Layers ; 71.2.2d Mica Group (Hydromica subgroup)
High temperature:
Low temperature mineral. At higher temperatures( 700C), micas can form in acid environments as long as excess potassium is available.
Geological context:
Illites are the dominant clay minerals in shales and mudstones, and they also occur in other sediments such as limestones. The illites of sediments may have been deposited as such after their formation by surface weathering of silicates, principally feldspars, but in some occurrences they are derived by alterationof other clay minerals, during diagenesis.
Dernière mise à jour le 25.01.2021