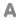Formula : CaCl2:H2O
Family:
High temperature:
Stable at room temperature. The structure is very responsive in temperature and/or water-vapor pressure
Geological context:
In the natural environment known as hydrophilite. Occurs as product from volcanic fumaroles.
Dernière mise à jour le 25.01.2021