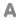Formula : CaO
Family:
Space group:
Isometric - Hexoctahedral H-M Symbol (4/m 3 2/m) Space Group: F m3m
Dana class:
4.2.1.5 (4) Simple Oxides (4.2) with a cation charge of 2+ (A++ O) (4.2.1) Periclase group (Isometric, Fm3m)
High temperature:
High temperature mineral around 900C and more. Also as byproduct from smelting.
Geological context:
In blocks of calcareous lava enclosed in the lava at Mt Vesuvius.
Dernière mise à jour le 25.01.2021